Published data show that, in mice, juvenile social isolation results in decreased sociability in adults and that this effect involves deep-layer neurons of the medial prefrontal cortex (mPFC). A new study expands our understanding of this mPFC-dependent circuit by identifying additional populations of neurons that modulate the impact of juvenile social isolation on social behavior later in life.
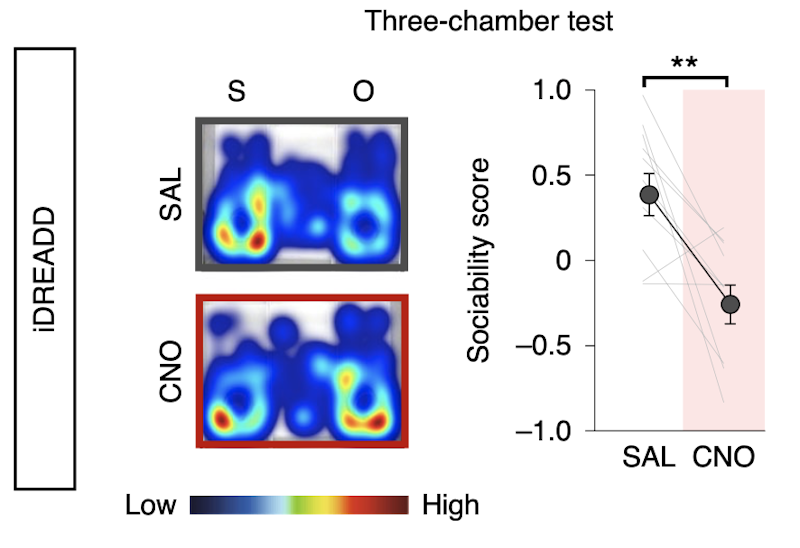
The work was supported in part by a Pilot Award to SFARI investigator Hirofumi Morishita. Morishita and his colleagues began their study by using c-Fos expression mapping to show that both mPFC deep-layer neurons and the posterior part of the paraventricular nucleus of thalamus (pPVT) were activated by exposure to a novel mouse. Using fiber photometry, they further showed that group-housed adult mice exposed to a novel mouse had stronger activation of mPFC→pPVT neurons expressing a calcium indicator than did adult mice that had been socially isolated as juveniles. Suppressing the activity of this circuit by chemogenetic or optogenetic means in adulthood resulted in the same sociability deficits induced by juvenile social isolation. Similarly, stimulation of the mPFC→pPVT circuit in adulthood led to a rescue of the sociability deficits. Finally, the authors found that juvenile isolation led not only to reduced excitability of mPFC→pPVT neurons but also to increased inhibitory input from somatostatin interneurons.
Given previous reports that many autism risk genes are highly expressed in deep-layer neurons of the mPFC, the authors suggested that it will be of great interest to determine if mutations in these genes lead to specific disruptions in the mPFC→pPVT circuit under study.
Reference(s)
A prefrontal-paraventricular thalamus circuit requires juvenile social experience to regulate adult sociability in mice.
Yamamuro K., Bicks L.K., Leventhal M.B., Kato D., Im S., Flanigan M.E., Garkun Y., Norman K.J., Caro K., Sadahiro M., Kullander K., Akbarian S., Russo S.J., Morishita H.


