Studies of mice carrying mutations in established autism spectrum disorder (ASD) risk genes have demonstrated that, at the cellular/circuit level, many show increased levels of neuronal activity due to a decrease in the amount of overall inhibition versus excitation at neuronal synaptic contacts within the brain’s cerebral cortex. This change in the excitation-inhibition (E/I) ratio has long been presumed to reflect a circuit-level cause of ASD dysfunction, but progress has been hampered by the lack of a consistent and rigorous definition of what ‘E:I imbalance’ actually means in the context of different brain regions and cell types.
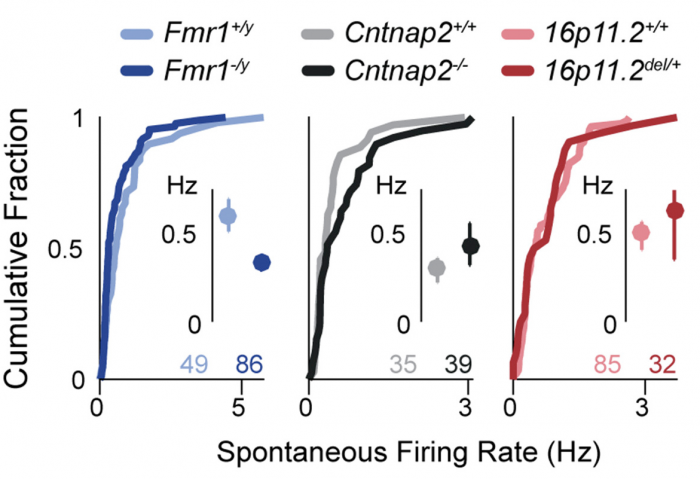
Now, SFARI Investigator Dan Feldman reports on the surprising finding that this increase in the E/I ratio may actually serve as a protective mechanism. In work supported by his SFARI Explorer and SFARI Pilot Award, Feldman’s laboratory assessed four distinct lines of mice carrying ASD risk mutations (Fmr1, Cntnap2, Tsc2 and 16p11.2 deletion mice), confirming that they demonstrate an increase in the E/I ratio in somatosensory cortex (S1). Importantly, these experiments were carried out in vitro, in brain slices, and in vivo, using single-unit recordings in S1 in response to whisker deflections, all on the same intracortical feedforward circuit. Feldman’s laboratory went on to show that, in all four cases, this increase in E/I was not detrimental, as had been previously presumed, but instead represents a homeostatic response that promotes a normalization of synaptic activity.
These findings shed new light on the role of E/I balance in ASD, suggesting that attempts to correct circuit function by altering the E/I ratio may actually prove harmful. The authors also speculate that the homeostatic response to normalized firing rates may drive ASD-related symptoms by compromising other aspects of circuit function.
Reference(s)
Increased excitation-inhibition ratio stabilizes synapse and circuit excitability in four autism mouse models.
Antoine M.W., Langberg T., Schnepel P., Feldman D.


