
Interventions for autism spectrum disorder (ASD) are mostly based on behavioral therapy. While drugs are available to treat comorbid behaviors, such as irritability and hyperactivity, pharmacological treatments targeting the core diagnostic symptoms of the disorder (e.g., social deficits) are lacking. Several factors have hindered the development of pharmacological therapies for ASD, including the diverse phenotype and genetic etiology of the disorder and the absence of biomarkers.
In the past years, a compound called ‘arbaclofen’ (R-baclofen, STX209) has received increasing attention as a potential therapeutic avenue. Clinical Research Associates, L.L.C. (CRA), an affiliate of the Simons Foundation, has acquired the rights to this drug and funded several studies testing its effects on the brain and behavior. This year, two large-scale clinical trials using CRA-sponsored arbaclofen are also planned to begin.
I recently spoke with Paul Wang, CRA Deputy Director, to discuss the use of arbaclofen as a potential drug treatment for autism and CRA’s plans for moving this research forward. The interview has been edited for clarity and brevity.
What is arbaclofen, and how does it act in the brain?
Arbaclofen is a GABAB receptor agonist. It is an enantiomer of racemic baclofen, which is, in fact, a mixture of R-baclofen and S-baclofen — two chemically identical forms that differ because they are mirror images of each other. What’s interesting is that almost all of the pharmacologic action of racemic baclofen resides in the R-enantiomer, and the S-enantiomer is basically inactive.
Racemic baclofen is approved by the United States (U.S.) Food and Drug Administration (FDA), European Medicines Agency and many other regulatory authorities around the world to treat spasticity — muscle stiffness that is often associated with multiple sclerosis and cerebral palsy. In Europe, it’s approved for that use not only in adults, but also in children. In the U.S., it’s not approved for use in children, but physicians have been using it off label to treat children with cerebral palsy down to age 2 and even younger for decades. So they have very deep clinical experience using racemic baclofen and understand its safety profile.
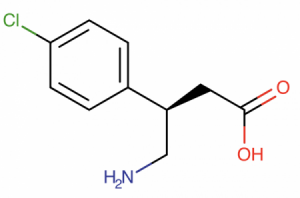
What is the rationale behind using arbaclofen in ASD?
We really only have hypotheses on why arbaclofen might work in individuals with ASD. One of these is based on the so-called E/I hypothesis of autism1, that there is an imbalance of excitatory to inhibitory neurotransmission, either because of too much excitatory or too little inhibitory transmission. So the idea is that, being a GABAergic drug, arbaclofen might rectify the balance. But GABAB receptors are widely distributed in the brain, so this is a hypothesis with very poor resolution.
Another rationale is based on behavioral rescue studies done in various mouse models of autism, including 16p11.2 deletion and Fmr1 knockout mice (a model for fragile X syndrome). Arbaclofen reversed cognitive deficits and improved social interactions in two lines of 16p11.2 deletion mice2, as well as rescued neuroanatomical and electrophysiological deficits3 and reduced social behavioral deficits in Fmr1 knockout mice4. Because a significant number of people with 16p11.2 deletion syndrome has a diagnosis of autism and almost all individuals with fragile X syndrome have some autism-related symptoms, it was hypothesized that arbaclofen might also be helpful to at least some people with autism.
There was also a lot of excitement around the mGluR theory of fragile X5, which posits that fragile X results from abnormalities in metabotropic glutamate receptor (mGluR) signaling. We hypothesized that the mGluR theory might also be relevant to at least a subgroup of ASD, even in the absence of a fragile X diagnosis. Arbaclofen is relevant to this hypothesis because presynaptic GABAB receptor activation can modulate mGluR signaling.
Clinical trials testing the efficacy of arbaclofen in individuals with autism were initially conducted by Seaside Therapeutics. What was the outcome of those studies?
The first clinical trials were sponsored by a biotech company, Seaside Therapeutics, that was founded by SFARI Investigator Mark Bear. It’s a company where I also worked as senior vice president for Clinical Development for about five years. During that time, we ran studies of arbaclofen in both individuals with fragile X syndrome and in individuals with autism not due to fragile X syndrome. In Seaside’s smaller-scale, shorter-duration trials, it looked like there were positive effects in individuals with fragile X syndrome in a double-blind, placebo-controlled, crossover trial6.
The question I think really is: Was that a good endpoint? Back then, I thought it was, but now I think it clearly is not.
We also conducted an open-label study in autism that showed improvement on a lot of different endpoints, including sociability7. But, of course, we know that open-label studies often show positive results that are not replicated in a controlled study. So Seaside Therapeutics went on to run three larger-scale trials, two of them in fragile X syndrome and one of them in autism. Unfortunately, none of these studies were positive on the primary endpoint8, 9, and in short, that led to the demise of Seaside Therapeutics. The investors did not feel that the data were promising enough for them to provide more investment money, and the company ceased operations.

What motivated CRA to support more studies of arbaclofen in ASD?
Interestingly, a number of academics and clinicians who participated in the Seaside trials felt that, even though the outcome was not positive, there were some promising signals. They thought the design of the study may have been inadequate and that a better designed study might show positive results on a primary end point.
The Seaside autism trial included 150 children age 5–21 years old, who were treated for 12 weeks with arbaclofen. The primary endpoint was the Social Withdrawal/Lethargy subscale of the Aberrant Behavior Checklist (ABC). The results showed that even though the participants on arbaclofen improved, those on placebo improved by almost exactly the same amount and actually slightly more. In other words, there was a very large placebo effect, and arbaclofen wasn’t doing better than that. The question I think really is: Was that a good endpoint? Back then, I thought it was, but now I think it clearly is not.
This endpoint had been chosen because risperidone and aripiprazole were approved by the FDA on a closely related endpoint from the same scale: the Irritability, Agitation, Crying subscale of the ABC. Unfortunately, we found out that it is very susceptible to placebo effects. However, on one of the key secondary endpoints, the CGI-S [Clinical Global Impression of Severity scale], we found that the improvement was much greater among the subjects who were treated with arbaclofen than subjects who were treated with placebo. But this was not the primary endpoint of the study.

The other thing that Seaside did was a post-hoc data analysis. While exploring the data to see if there were subgroups who showed greater improvement, we noted that participants who were higher functioning, especially those who were more verbal, showed greater evidence of change than others. We don’t know if that’s because the drug actually works better in those children. We have no biological hypothesis for why it would work in one group and not the another. We really think it is a function of the endpoint, of the assessment being more sensitive to change in individuals who are more verbal. And so, those post-hoc analyses have really driven the design of the studies that are being contemplated now, one in Europe and one in Canada.
How will these new studies differ from the previous ones?
I think there are three major differences between the studies that are being planned now versus the Seaside study. First, the current studies will only enroll more verbal subjects, and second, the primary endpoint will be different. Instead of the ABC Social Withdrawal subscale, these studies will use the Vineland Adaptive Behavior Scales-II Socialization subscale score. We believe this subscale is less susceptible to placebo effects; indeed, this appeared to be the case in the Seaside study, and it has also been observed in trials that other investigators have run to look at other treatments for autism. And it is also a clinically meaningful endpoint, a gold standard of psychoeducational assessment.
Lastly, these studies are both slightly longer. The Seaside study ran for twelve weeks, and these studies will last sixteen weeks; that’s because, on the CGI-S in the Seaside study, it looked like the improvement had not reached a plateau at the twelve-week point. So we believe there is the possibility that improvement could continue beyond twelve weeks.
We would like to see that participants improve in their socialization on arbaclofen more than they do on placebo.
You mentioned two upcoming clinical trials, one in Europe and one in Canada.
The European arbaclofen trial is part of a very large project called Autism Innovative Medicine Studies-2-Trials (AIMS-2-Trials), which is the second five-year phase of an Innovative Medicines Initiative project focused on autism. The first five-year project on autism was called European Autism Interventions – A Multicentre Study for Developing New Medications (EU-AIMS). The AIMS-2-Trials was recently approved by the European Commission and has a much greater focus on treatment, whereas EU-AIMS was focused more on phenotyping and understanding the natural history of disease and establishing animal and cellular models of autism. The project leads for AIMS-2-Trials are Declan Murphy (King’s College London) and Will Spooren (F. Hoffmann-La Roche) and the Principal Investigator for the arbaclofen trial is Celso Arango (Hospital General Universitario Gregorio Marañón).
In Canada, the study is being funded through the Canada Brain Research Fund — a partnership between private and federal funders — and a provincial program called the Provence of Ontario Neurodevelopmental Disorders (POND) network. The Principal Investigator is Evdokia Anagnostou (Holland-Bloorview Kids Rehabilitation Hospital and University of Toronto).
The European and Canadian investigators have been working together very closely to try to make their studies as similar as possible to each other, as their intent really is to make these studies so similar that the data can be pooled into a single analysis. The funding in Europe only allows a study of about 130 participants, and the funding in Canada only allows about 90 participants. The Seaside study was 150, so we know that each of those is powered slightly less than this, but together, they would have greater power. That’s why they want to align them as closely as possible.
What are the desirable outcomes of these trials?
You know, we always start with safety. In the Seaside study, arbaclofen was very well tolerated; there were no serious adverse events. We expect — and hope — that this will be true also in the European and Canadian studies. Of course, we would like to see that participants improve in their socialization on arbaclofen more than they do on placebo. We also hope that if there is a subset of participants who respond better than others, we might be able to identify them. And again, those individuals may be determined by a demographic variable, maybe age; maybe even within the more verbal group, there are other distinctions that could be drawn.
What are the most interesting aspects of these new trials, in your opinion?
These studies will be also assessing electrophysiological biomarkers through electroencephalogram (EEG) and event-related potential (ERP) measures. So we are very excited about the possibility of identifying EEG and ERP biomarkers. Honestly, I am excited that these academics thought there was sufficiently interesting data on arbaclofen that they wanted to conduct more studies of this. We did not solicit them to run these studies; they were motivated themselves to run them and came to us to ask if we would supply the drug, and we are very happy to do so.
Is CRA working on any other projects besides arbaclofen?

All of our projects at CRA right now center on arbaclofen. There are a few more human arbaclofen studies for which we have provided not only the drug but also funding support.
One is a brain imaging study led by Timothy Roberts (Children’s Hospital of Philadelphia). He is running a double-blind placebo-controlled study comparing a low and a high dose of arbaclofen to placebo and using magnetoencephalography to assess auditory brain responses in children with ASD. He is also conducting magnetic resonance spectroscopy (MRS) scans to look at the balance between glutamine/glutamate to GABA.
Another study is being conducted by Grainne McAlonan (King’s College London). She is using EEG and ERP to look at responses to visual stimuli after arbaclofen treatment. She is also conducting MRS scans, using the same dosing scheme as Tim Roberts. Her study participants are adults both with and without ASD.
We’re also funding Caroline Robertson (Dartmouth College) to conduct a study that examines deficits in perceptual suppression in binocular rivalry and underlying alterations in GABA signaling in autism. She is now going to examine the effects of arbaclofen on binocular rivalry, in a double-blind placebo-controlled study looking at only one dose of arbaclofen in healthy young adults.
We’d love to hear from investigators who have ideas on how arbaclofen might be used...
Finally, we have provided arbaclofen and funding support to a number of investigators who are conducting basic research studies in animals, most recently to four different investigators who are studying various mouse models of the 16p11.2 deletion. Preliminary findings from their studies were presented at the International Society for Autism Research (INSAR) meeting earlier this year, and their most recent findings will be presented at Neuroscience 2018.
What are CRA’s future directions?
We will be working with the European and Canadian investigators closely to make sure these trials run as efficiently and rigorously as possible. I don’t think we will be sponsoring any large-scale clinical study of arbaclofen in autism until we know the results of these two trials. But we are very interested in the possibility that arbaclofen can be used in other contexts. It does seem to be a well-tolerated drug, it’s GABAergic, and, as we discussed, there is much interest around E/I hypotheses of autism. In particular, we would be interested in considering using arbaclofen as a tool to study E/I-related phenomena and potential biomarkers for E/I imbalance. We’d love to hear from investigators who have ideas on how arbaclofen might be used either in animal or human studies and are open to the possibility of providing the drug for their studies.
References
- Rubenstein J.L. and Merzenich M.M. Genes Brain Behav. 2, 255-267 (2003) PubMed
- Stoppel L.J. et al. Neuropsychopharmacology 43, 513-524 (2018) PubMed
- Henderson C. et al. Sci. Transl. Med. 4, 152ra128 (2012) PubMed
- Qin M. et al. Int. J. Neuropsychopharmacol. 18, pii: pyv03 (2015) PubMed
- Bear M.F. et al. Trends Neurosci. 27, 370-377 (2004) PubMed
- Berry-Kravis E.M. et al. Sci. Transl. Med. 4, 152ra127 (2012) PubMed
- Erickson C.A. et al. J. Autism Dev. Disord. 44, 958-964 (2014) PubMed
- Veenstra-VanderWeele J. et al. Neuropsychopharmacology 42, 1390-1398 (2017) PubMed
- Berry-Kravis E. et al. J. Neurodev. Disord. 9, 3 (2017) PubMed
- Gandal M.J. et al. Biol. Psychiatry 68, 1100-1106 (2010) PubMed
- Roberts T.P. et al. Autism Res. 3, 8-18 (2010) PubMed


